this post was submitted on 14 Sep 2024
20 points (95.5% liked)
Weather and Meteorology
174 readers
1 users here now
Hope to expand on this later. A community for discussing the weather (very UK), amateur meteorology, and moaning it's too hot/cold/wet/dry/mild.
founded 1 year ago
MODERATORS
you are viewing a single comment's thread
view the rest of the comments
view the rest of the comments
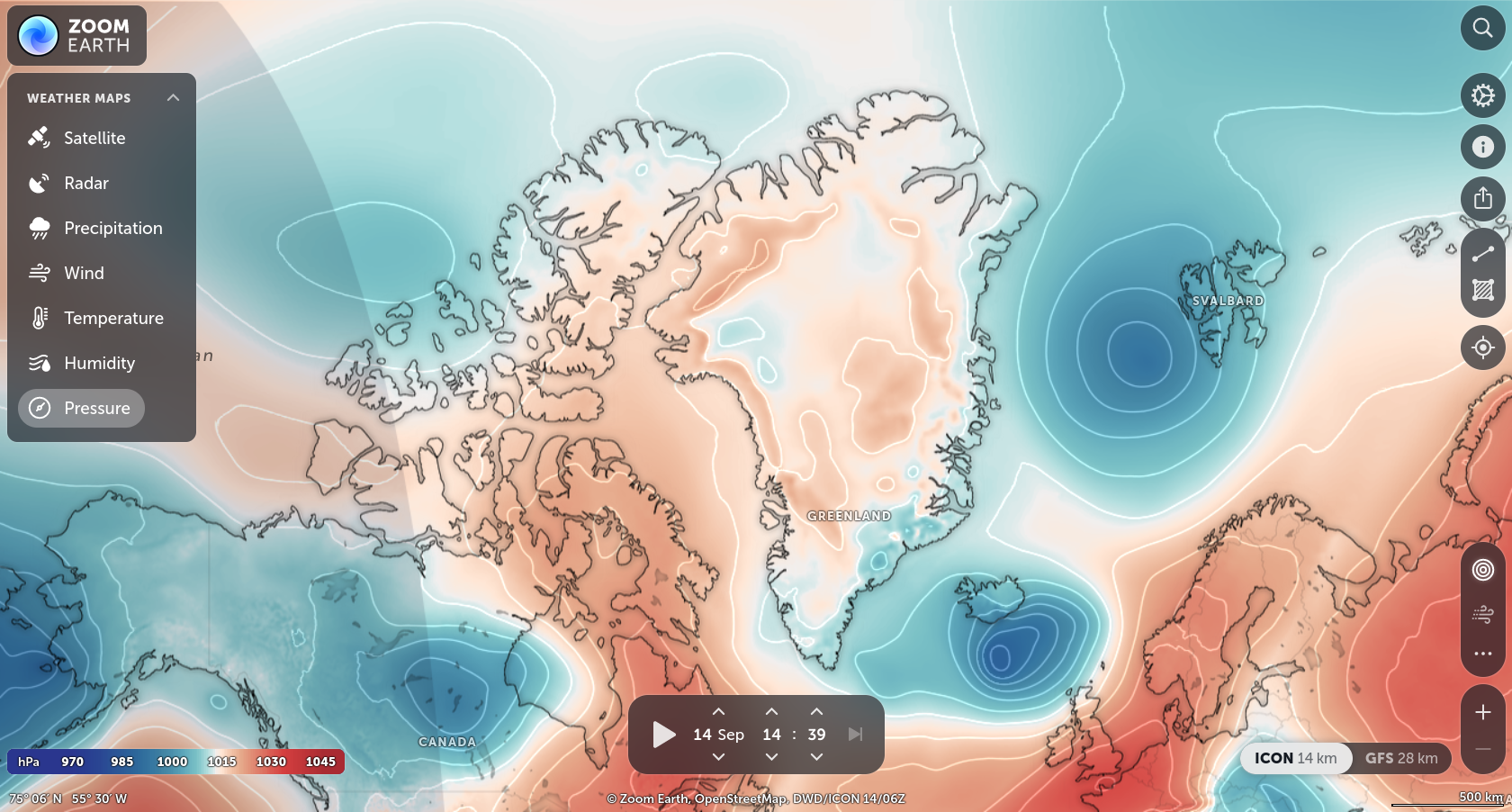
Low temperatures are associated with lower barometric pressure. Temperature and air pressure are proportional. You might be confusing density with air pressure?
The way it is taught in many Earth Science text books, is that if you have two similar volumes of air, but the first is colder than the second, the first will have a higher density, and therefore a larger mass, and therefore a higher air pressure (e.g. at the surface).
Following this logic, you expect higher pressure in colder areas, as long as the volume of air (the height of the air column) is the same. I think the answer to my question has to do with the latter: the atmosphere is less thick at the poles. As a result, despite the much lower temperatures, the air pressure at the poles is generally lower than (for example) at the subtropical highs.
the complication is that in meteorology, the volume of gas does not remain the same! if you're changing the mass, temperature, density, and pressure of a parcel of air, you definitely can't assume that the volume is constant
it's good to use a different ideal gas equation, instead of PV = nRT (pressure x volume = n * R * Temperature)
we meteorologists tend to stick to unit masses, and use: Pressure = density ×R×T, instead
i.e. when temperature decreases, pressure decreases
Also, the formula Pressure = density x R x T doesn't show that pressure decreases as temperature decreases, since a temperature decrease also increase the density?